
Now Enrolling: The CompassHER2 pCR trial is determining if certain patients with early-stage HER2 positive breast cancer may benefit from reduced chemotherapy
June 7, 2020
Cancer treatment de-escalation: improving cancer care by tailoring treatment
June 9, 2020Now Enrolling: The DECREASE trial seeks to decrease treatment side effects – and improve quality of life – for people with early-stage anal cancer

Cancer can be difficult to talk about – and when cancer involves parts of the body that are intimate or sensitive, it can be even more challenging to discuss. Still, it’s important to try, so that people living with these cancers might feel less alone or self-conscious. ECOG-ACRIN Cancer Research Group (EA) leads clinical trials for many different types of cancer, including those that are especially sensitive. EA recently opened the DECREASE trial, also known as EA2182, for patients with early-stage anal cancer.
There are different types of anal cancer, but the most common is squamous cell carcinoma, which begins in the outer lining of the anal canal. Patients who have early-stage disease do exceptionally well with the standard treatment and have a high five-year survival rate. Standard treatment includes chemotherapy and radiation (or chemoradiation) for a total of 28 treatment sessions.
Right now, many patients with early-stage disease receive the exact same amount of chemoradiation as those with more advanced disease. That means early-stage patients also experience the same likelihood of possible side effects related to bowel, urinary, and sexual function. However, researchers suspect that early-stage patients may do just as well with lower doses of chemoradation – lower doses that may mean fewer side effects.
The DECREASE study is evaluating whether lower doses of chemoradiation will be able to effectively treat early-stage anal squamous cell carcinoma while also decreasing side effects. Through this study researchers hope to establish a treatment standard that is individualized for patients with early-stage disease. They also hope to improve both short- and long-term quality of life for these patients.
 “Hopefully this study will take us into a new era of personalized treatment for anal cancer,” said study chair Jennifer Dorth, MD (pictured) of Case Western Reserve University. “We know that chemoradiation works well, so now we need to move our treatments into the modern era, to personalize based on tumor size and to improve radiation planning and delivery.”
“Hopefully this study will take us into a new era of personalized treatment for anal cancer,” said study chair Jennifer Dorth, MD (pictured) of Case Western Reserve University. “We know that chemoradiation works well, so now we need to move our treatments into the modern era, to personalize based on tumor size and to improve radiation planning and delivery.”
Patients may be eligible for this trial if they are 18 years of age or older, and have not yet received treatment for their early-stage anal squamous cell carcinoma. Participants will be randomly assigned to one of two study groups. The first group will receive standard-dose chemoradiation therapy (Group 1 below), while the second group will receive lower-dose chemoradiation therapy (Group 2). After finishing treatment, patients in both groups will be followed for up to five years.
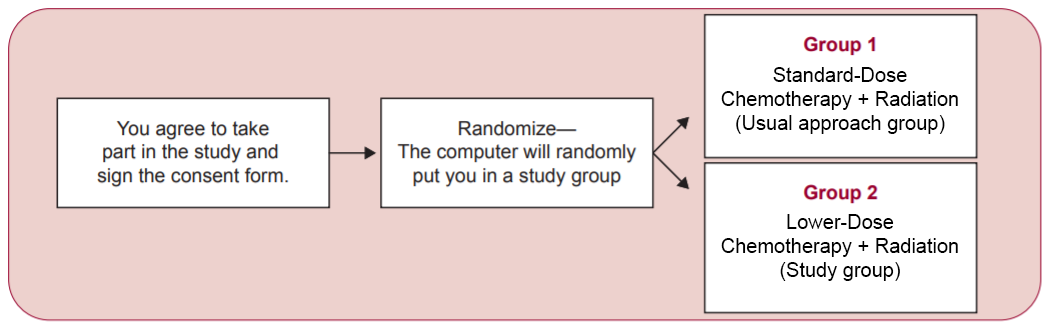
“We have good reason to believe, based on smaller studies, that this approach will work and the cure rates will be maintained in the lower-dose group,” said Dr. Dorth. “But we need to confirm that this approach is effective with the DECREASE study before we can define a new treatment standard.”
Learn more about the DECREASE trial.

