Ongoing Trial: EA3161 is testing maintenance immunotherapy versus observation in patients with HPV-related throat cancer
June 6, 2020
Now Enrolling: The DECREASE trial seeks to decrease treatment side effects – and improve quality of life – for people with early-stage anal cancer
June 8, 2020Now Enrolling: The CompassHER2 pCR trial is determining if certain patients with early-stage HER2 positive breast cancer may benefit from reduced chemotherapy

The more researchers learn about different types of cancer, the more they can improve treatment. However, improved treatment does not always take the same form. Sometimes, it means adding a new type of therapy to the current treatment, like a new drug. Sometimes, it means increasing the amount of the current treatment. And sometimes, it even means reducing the amount of the current treatment to help minimize side effects and optimize quality of life.
 The purpose of the CompassHER2 pCR study (also known as EA1181), led by Nadine Tung, MD (pictured) of Beth Israel Deaconess Medical Center, is to find out if some patients with early-stage HER2 positive breast cancer can safely be treated with less chemotherapy than is generally used. Currently, these patients receive limited chemotherapy before surgery, surgery, and then more chemotherapy after surgery. However, several small studies suggest that patients who receive chemo before surgery and have no remaining tumor at the time of surgery do very well without chemo after surgery. In fact, for these patients, chemotherapy after surgery may provide no benefit at all while still resulting in additional chemotherapy side effects.
The purpose of the CompassHER2 pCR study (also known as EA1181), led by Nadine Tung, MD (pictured) of Beth Israel Deaconess Medical Center, is to find out if some patients with early-stage HER2 positive breast cancer can safely be treated with less chemotherapy than is generally used. Currently, these patients receive limited chemotherapy before surgery, surgery, and then more chemotherapy after surgery. However, several small studies suggest that patients who receive chemo before surgery and have no remaining tumor at the time of surgery do very well without chemo after surgery. In fact, for these patients, chemotherapy after surgery may provide no benefit at all while still resulting in additional chemotherapy side effects.
In the CompassHER2 pCR study, patients will receive one chemotherapy drug (a taxane) and two antibody HER2-targeted drugs (trastuzumab and pertuzumab) for about 12 weeks before surgery. Then, patients who have no remaining cancer found in their breast or lymph nodes at the time of surgery will have surgery without any additional chemotherapy afterward. However, they will continue to receive treatment with HER2-targeted drugs (trastuzumab and pertuzumab) for one year, plus routine care with radiation therapy and anti-estrogen medications if needed.
Patients who do have tumor left in their breast at the time of surgery will be counseled by their physician on the best course of treatment after surgery. This could include additional chemotherapy. All patients on the study will be followed for up to 15 years. Patients will also be asked to provide blood samples and tissue from their tumor, already collected as part of routine care, for the study of laboratory markers that could help explain why certain tumors respond better or worse to treatment.
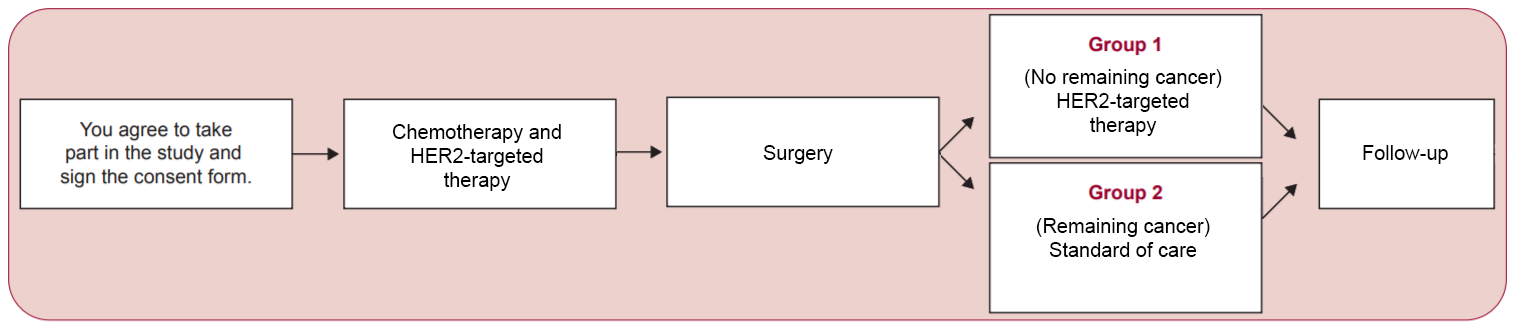
Ultimately, CompassHER2 pCR should establish if patients who have no residual cancer at surgery and receive no chemotherapy after surgery will have good outcomes without their cancer coming back. This type of “personalized” or “precision medicine” study will teach doctors how to make sure they give the right amount of treatment to optimize patients’ chances of doing well while simultaneously reducing their risks of complications from therapy.
Learn more about the CompassHER2 pCR trial.

