Trial Results: ECOG-ACRIN plans to conduct a randomized phase 3 trial aimed at sparing people with oropharynx cancer unnecessary side effects from treatment, based on the recent results of phase 2 study E3311
June 6, 2020
Now Enrolling: The CompassHER2 pCR trial is determining if certain patients with early-stage HER2 positive breast cancer may benefit from reduced chemotherapy
June 7, 2020Ongoing Trial: EA3161 is testing maintenance immunotherapy versus observation in patients with HPV-related throat cancer
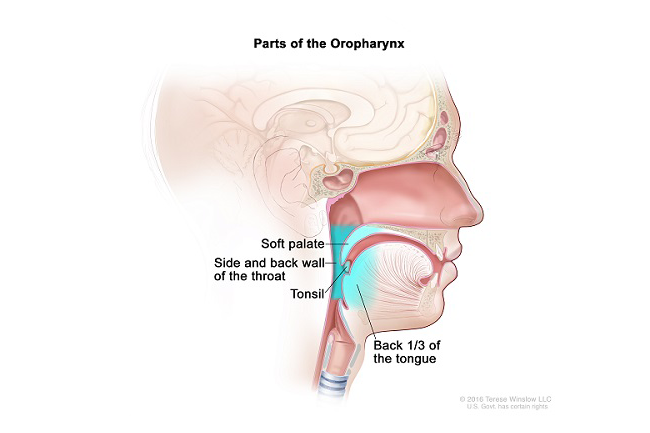
There are two distinct types of head and neck squamous cell cancer: those unrelated to human papillomavirus (HPV), called HPV-negative, and those related to HPV, called HPV-positive. HPV is commonly recognized as a risk factor for cervical cancer – but it is also a significant risk factor for oropharyngeal (or throat) cancer. In fact, HPV-positive throat cancer (specifically cancer of the base of the tongue or tonsils) is now the number one HPV-related cancer in the United States.
Patients with HPV-positive throat cancer who have a low risk of it returning or spreading to other parts of the body often respond extremely well to treatment. At five years after diagnosis, more than 95 percent of them are still alive. However, some patients do not have such a good outcome, usually because they have a higher stage disease or a history of smoking. These patients need better treatment options. They have what is called “intermediate risk” HPV-positive disease, and they only have a 60 – 70 percent rate of survival at five years after diagnosis.
Clinical trial EA3161 aims to address this problem and improve outcomes for patients with intermediate-risk disease. The study is comparing the usual (standard) treatment approach, which is a combination of chemotherapy and radiation, to the usual treatment plus a drug called nivolumab, a drug that allows the immune system to attack the cancer (immunotherapy). Nivolumab is given to patients after their initial treatment to hopefully increase the chances of the cancer not coming back and prolonging patients' lives – an approach called “maintenance therapy.”
 “There has been a precedent in oncology, specifically in lung cancer, that when we add a maintenance therapy approach for patients who have received radiation therapy, we do have an advantage in outcome,” said study chair Nabil Saba, MD (pictured) of Emory University’s Winship Cancer Institute. “Since 2016, the FDA has approved two immunotherapy drugs for the treatment of advanced head and neck cancer – drugs that have significantly changed the way we treat advanced, metastatic head and neck cancer.”
“There has been a precedent in oncology, specifically in lung cancer, that when we add a maintenance therapy approach for patients who have received radiation therapy, we do have an advantage in outcome,” said study chair Nabil Saba, MD (pictured) of Emory University’s Winship Cancer Institute. “Since 2016, the FDA has approved two immunotherapy drugs for the treatment of advanced head and neck cancer – drugs that have significantly changed the way we treat advanced, metastatic head and neck cancer.”
These drugs have led to long-term control of cancers in some patients, and improvement in survival, while also maintaining good quality of life. Now, Dr. Saba and the team at ECOG-ACRIN want to see if immunotherapy can help patients with less advanced disease who are newly diagnosed.
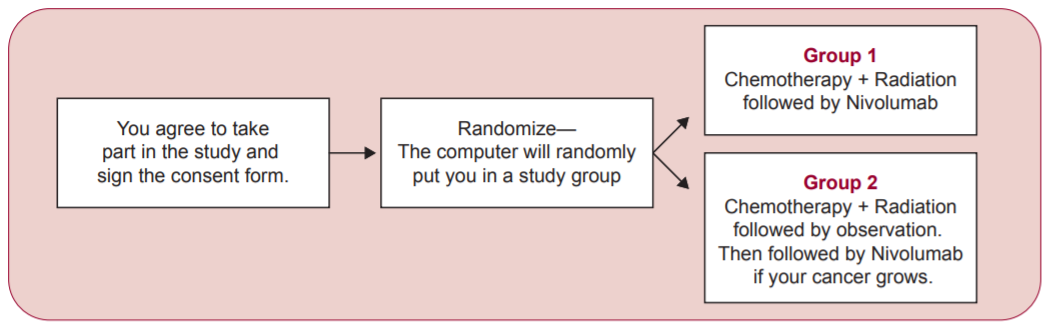
Patients may be eligible for this trial if they are 18 years of age or older and have not had previous treatment for their HPV-positive throat cancer. All patients on the trial will receive standard chemotherapy and radiation; then, patients will either receive maintenance nivolumab for one year (Group 1 below) or they will simply be followed by their physician to see if the cancer returns (Group 2). If a patient’s cancer does come back, they may cross over to the nivolumab group to see if the drug helps them.
“If this is a positive trial, it could have a huge impact on the way we practice for this group of patients,” said Dr. Saba. “It could be a major game changer for treating intermediate-risk, HPV-positive disease.”

