
Trial Results: New TAILORx data shows chemotherapy benefit for women at high risk of breast cancer recurrence
February 12, 2020
Ongoing Trial: EA3161 is testing maintenance immunotherapy versus observation in patients with HPV-related throat cancer
June 6, 2020Trial Results: ECOG-ACRIN plans to conduct a randomized phase 3 trial aimed at sparing people with oropharynx cancer unnecessary side effects from treatment, based on the recent results of phase 2 study E3311
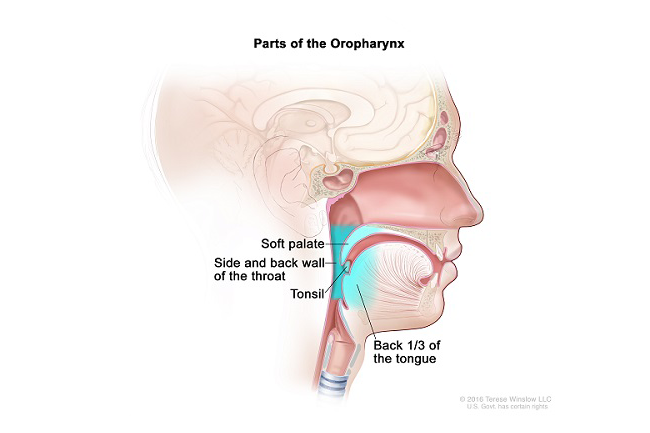
The overall intent of the recently completed phase two trial E3311 was to gather essential data for the design of a future trial. ECOG-ACRIN plans to pursue the current data with a randomized phase three trial of transoral robotic surgery (TORS)-based treatment compared with conventional chemotherapy and radiation.
A lump in the neck and a sore throat are the most common signs of oropharynx (throat) cancer, a disease in which cancer cells form in the tissues around the tonsils and the base of the tongue. About 60% of all oropharynx cancers are associated with human papillomavirus (HPV) infection.
HPV-related throat cancer is increasing rapidly, especially in people under the age of 45. Standard treatment involves one or more of the following: surgery, chemotherapy, and radiation therapy. Many hospitals and cancer centers offer TORS, a type of surgery that uses endoscopy and two robot-controlled instruments to remove cancer cells and spare patients’ vital throat functions such as swallowing.
This type of cancer usually responds well to treatment, especially in people who have a low level of risk that it might return or spread to other parts of the body after treatment (this is called their recurrence risk). At five years after diagnosis, more than 95% of patients are still alive. However, other patients do not have such a good outcome, usually because they have a higher stage of the disease, a history of smoking, and/or the tumor cells have a certain mutation. These patients have an intermediate (medium) risk of recurrence, and for them, the chance of being alive at five years is 60-70 percent.
The younger age of people with HPV-related throat cancer, combined with their good prognosis, means that increasing numbers of patients will live for several decades with the side effects of the toxicities of chemotherapy and radiation. About 10 years ago, head and neck cancer researchers started conducting clinical trials that explore the concept of treatment optimization, which can include de-escalation. De-escalation, or de-intensification, is an effort to reduce the intensity of treatment without sacrificing effectiveness.
E3311 is such a study. Its results offer new information about using less treatment in patients with oropharynx cancer and a medium risk of recurrence. First, this trial found a better way to assess each patient’s individual risk. The new method uses tumor testing along with patient characteristics to measure the level of risk: low, intermediate (medium), or high. Physicians may safely consider offering patients less therapy if their risk of recurrence is low. But what about patients with intermediate, or medium risk?
The E3311 study found a de-escalation strategy for medium-risk patients. The question was: Could this group of patients forego chemotherapy altogether and receive a lower dose of radiation than is standard, and still have good outcomes? To find the answer, the trial enrolled 353 patients. All had TORS first with surgeons who were credentialed cancer specialists trained to perform TORS. Tumor tissue was collected during surgery. Using the results of their tumor test and other information, patients were then treated based on their recurrence risk. Low-risk patients had no chemotherapy or radiation after surgery. High-risk patients received standard chemotherapy and radiation after surgery.
For patients in the medium-risk group, the trial studied two doses of radiation, both lower than standard. After surgery, these patients were randomized to receive one or the other dose without chemotherapy.
The new method of measuring risk proved to work well. Across all four groups, two-year progression-free survival rates were above 90% and there was no excess of local recurrences by reducing radiation or chemotherapy. Local recurrences refer to cancer that has come back at or near the same place as the original (primary) tumor. For the two medium-risk groups, two-year progression-free survival was 95.0% for a 50 Gy dose of radiation, and 95.9% for a 60 Gy dose.
 “For medium-risk patients, reduced-dose postoperative radiation therapy without chemotherapy appears sufficient,” said lead researcher Robert L. Ferris, MD, PhD, director, UPMC Hillman Cancer Center in Pittsburgh, and a surgical oncologist specializing in head and neck cancer (pictured). “Two years after finishing treatment, this group had better outcomes than the group on the usual high-dose radiation plus chemotherapy ... preserving patients’ throat function and sparing them unnecessary short- and long-term side effects.”
“For medium-risk patients, reduced-dose postoperative radiation therapy without chemotherapy appears sufficient,” said lead researcher Robert L. Ferris, MD, PhD, director, UPMC Hillman Cancer Center in Pittsburgh, and a surgical oncologist specializing in head and neck cancer (pictured). “Two years after finishing treatment, this group had better outcomes than the group on the usual high-dose radiation plus chemotherapy ... preserving patients’ throat function and sparing them unnecessary short- and long-term side effects.”
For patients with low-risk disease, two-year progression-free survival was favorable with observation alone (93.9 percent). For the high-risk group, two-year progression-free survival was 90.5% with a radiation dose of 60-66 Gy and weekly cisplatin chemotherapy.
The American Society of Clinical Oncology (#ASCO20) highlighted these results at this year’s annual meeting. “Transoral resection followed by low-dose radiation alone, without chemotherapy, is safe in patients with intermediate-risk locally advanced oropharynx cancer, with very good outcomes,” said Dr. Ferris. “These results present a promising de-intensification approach.”
The ECOG-ACRIN Cancer Research Group designed and led the trial with funding from the National Cancer Institute, part of the National Institutes of Health.

