Now Enrolling: The DIRECT study is testing how well FDG-PET/CT imaging can predict if treatment is working prior to surgery for patients with HER2-positive breast cancer
July 19, 2023
Trial Results: ECOG-ACRIN research round-up – Fall 2023
October 9, 20237 ways advocates have enhanced ECOG-ACRIN’s cancer research program

For people unfamiliar with cancer research—and, in some cases, even for those in the field—the role of the cancer research advocate may be unclear. Within ECOG-ACRIN Cancer Research Group (ECOG-ACRIN), a committee of engaged advocates works to ensure patients have a voice and are represented at every stage of the clinical trial process.
At the Spring 2023 ECOG-ACRIN Group Meeting, members of the Cancer Research Advocates Committee reflected on key accomplishments. Not only are these achievements, summarized below, important contributions to cancer research; they illustrate the vital role of research advocates in advancing cancer care.
- The committee helped to ensure that every ECOG-ACRIN clinical trial protocol document includes a patient thank you letter.
One of the most effective ways to emphasize the importance of clinical trials and ensure participant retention is to simply say “thank you.” Each ECOG-ACRIN clinical trial protocol document (the detailed study plan) includes a thank you letter that physicians and research teams can adapt and send to their patients who participate in the trial. This small gesture can help honor patients and reinforce the importance of their participation. The goal is to show participants that researchers value their decision to help advance cancer research by joining a clinical study. - Research advocates serve on nearly all of ECOG-ACRIN’s committees, subcommittees, and working groups.
ECOG-ACRIN comprises nearly 40 scientific committees, subcommittees, and working groups that are responsible for clinical trial development and implementation. These groups are organized by cancer type (e.g., breast, melanoma) or by specialty, and members include physicians, translational scientists, and associated research professionals. Most committees also have at least one advocate representative whose role is to share patients’ perspectives and experiences. A few topics advocates can uniquely address include:-
-
The practical aspects of participating in a clinical trial and what might be attractive and/or a barrier for potential participants
- The informed consent document and if it is understandable to a lay audience
- This document outlines the study’s details and possible benefits and risks; every patient who wishes to participate in the study must sign it before enrolling
- Patient educational materials and if they are understandable to a lay audience
-
-
- ECOG-ACRIN is developing lay-friendly summaries of the results of clinical trials for dissemination to patients.
Researchers traditionally present the results of clinical trials at medical conferences and in scientific journals—but these presentations and publications use technical language designed for specialists. Providing lay-friendly summaries of results that are accessible to general audiences helps increase knowledge of the latest in cancer research across patient communities. Sharing these summaries is also a great way to acknowledge trial participants for their contributions to research and to improving care for future patients. - Advocates may provide direct feedback to study chairs on new trial concepts.
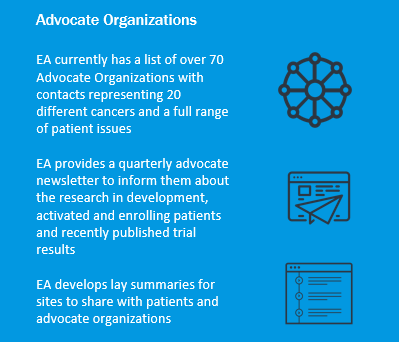
Speaking up during large scientific committee meetings can be intimidating, so the Cancer Research Advocates Committee established a process by which advocates can connect with committee and study chairs individually to share thoughts and ask questions about new clinical trials that are being considered. This helps facilitate information exchange in a setting that may be more inviting and comfortable than a full committee meeting. The goal is to give advocates an enhanced ability to express concerns, clarify research goals, and represent patients. - The committee created an infographic on the role of cancer research advocates and shared it with investigators.
See below for just a couple of highlights from the infographic:

- The committee developed an orientation program to train new ECOG-ACRIN advocates and familiarize them with the Group.
This orientation program sets up new advocates for success at ECOG-ACRIN. The program involves an introduction to ECOG-ACRIN and various National Cancer Institute (NCI) research areas such as the National Clinical Trials Network (NCTN) and NCI Community Oncology Research Program (NCORP). Among the overviews:-
- Clinical trial basics
- The protocol development process
- ECOG-ACRIN’s patient education and awareness resources
-
- During development of the NCI-MATCH precision medicine trial, advocate input led to the removal of a drug holiday for participants.
As investigators and advocates designed the NCI-MATCH precision medicine trial, which primarily enrolled patients with advanced cancers, there was discussion of including an intentional break from taking medication for a period of time—something doctors informally refer to as a “drug holiday.” The purpose of the drug holiday was to help show if a patient’s cancer was responding to the treatment.Advocates involved in these discussions raised concerns immediately. A case was made that if patients were forced to halt potentially life-prolonging treatment, they would be reluctant to join the study. Ultimately, the study team listened and removed the drug holiday. The result? NCI-MATCH accrued patients at a rapid pace, enrolling 800 participants in its first three months while investigators only estimated 150. Though several factors contributed to this success, the incorporation of advocate input is certainly relevant.
These seven accomplishments, while significant, by no means represent all that the committee has achieved since its inception. And, there is still plenty of work to do to help ensure the patient voice is represented throughout the entire clinical trial process. ECOG-ACRIN looks forward to continued collaboration with its research advocates to plan and conduct patient-centric clinical trials.